For amost over 150 years, the periodic table has been one of the most valuable tools for both chemists and physicists around the world. It is one of the first things that children in school come into contact with when starting their chemistry course, and one of the chemistry’s most “iconic” things. But is it the best deptiction of the known elements?
Since its conception by Gregor Mendeleyev there has been gaps in the table due to its design and ordering atoms by their respective number of protons. What alone means that it was designed to be flexible. Regularly new additions are made, the most recent ones as late as 2016.1 Any chemist can also easily imagine the table would expand if we discovered the fabled 9th period (probably in the upcoming 15 years). The structure is really easy to comprehend, and even at high school levels, things like periodic trends are introduced. These trends (although with some exceptions) hold up pretty well. Most of the strong points of the Mendeleyevs invention origin from the ordering elements by increasing number of protons.
So why reinvent the wheel? There is a couple of caveats that don’t tell a story of periodic table greatness. First of all (literally) - hydrogen. This element is categoraized along with alkali metals, even though it does not share any of their properties. My students asked me time and time again if it shouldnt be placed in group 17.2 Another case can be made of a couple of odd elements in the d block - mercury, gold and their neighbours. Due to their mass, some relativistic effects take place (giving lead its liquid state and gold its color).
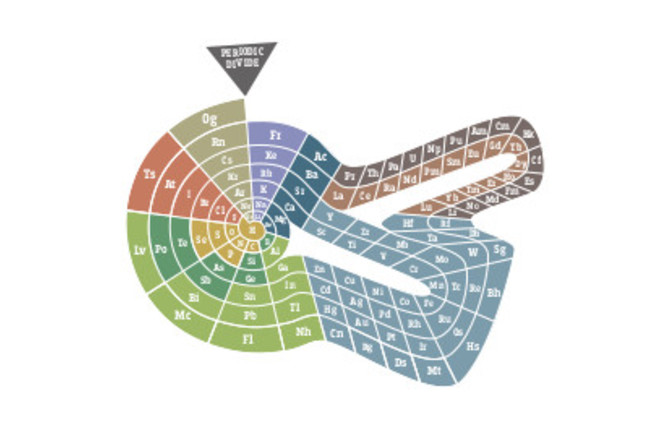 |
|---|
| Theodor Benfey’s “periodic snail. |
When the well-established 18-column table was thriving in scientific books, other scientists tried to best its utility. Some of the more known designs include a “periodic snail”, made by German Theodor Benfey, emphasizing continuity of the elements, completely ditching the breaks that are in the original version. Another strong contender is a left-step periodic table made by Frenchman Charles Janet, has the orbital groups separated - s, p, d and f blocks have their own subtables. This is arranged in such a way that allows for the continuity of the orbitals - so somtheing some students are struggling with. This one is not without flaws either - helium is clumped in the same group as alkaline earth metals and alkali metals - not very accurate in terms of its behaviour. Some more odd designs took periodic table to another dimension - literally. Canadian chemist named Fernando Dafur created a 3D pine-like structure, there each period is another “branch”. This design highlights relationships between the lements better, but the design itself - not legible.
There are many ways to imagine all the elements out there, but, I do not think how we render periodic table makes a big difference for experienced chemist. But for the sake of studying it for the first time, there could be some easier to master alternatives (looking at you, left-step!).
sources
- Trouble in the periodic table
- The Search for the Perfect Periodic Table
- 20 Years of Discovery: How Has the Periodic Table Changed Over Time?
footnotes
Footnotes
-
With a total of 5 additions since year 2000 - elements 114 through 118. ↩
-
That would make sense - Hydrogen can gain an electron, even though it prefers to lose it. I know that the placement is due to 1st period only having s orbital fitting 2 electrons, but it bothers me as much as it does my students. But a discussion about the chemical nature of hydrogen should take place in entirely different topic. ↩